All too often, stationary batteries are purchased many months before they are destined to be put into service and they are put in temporary storage. This storage can be either away from or at the installation location. Also, sometimes batteries are installed at their intended location but are not connected to the charging system. This paper will detail how batteries should be stored in such a manner that they will not degrade or suffer permanent damage. Freshening charging will be discussed and temperature, humidity, and other environmental factors will be addressed. Additionally, the correct charging voltage and various compensation factors will be given. Some practical examples will be shown.
Introduction
Throughout my career, I have witnessed the good, the bad, and the plain ugly storage of stationary batteries. This paper will look at the proper short and long term storage. There are multiple reasons for taking certain actions during battery storage, and these will be detailed. The correct charging method will be given and how to adjust for temperature variants. The length of time that various batteries can be stored will also be discussed. A long-term myth will be dispelled. And since there does not appear to be any definitive standards or guides for storing stationary batteries, I hope this paper will somewhat fill that gap.
Terminology, Limitations, and Applicability.
In this document, the term “battery” refers to a collection of single or multi-cell units. This paper is only applicable to stationary batteries and only addresses Vented Lead-Acid (VLA) and Valve-Regulated Lead-Acid (VRLA) chemistries. It is applicable to all those who are responsible for the project planning, transportation, storage, installation, and maintenance of these batteries. End users can include any industry segment that uses these stationary batteries for reserve power. This is also referred to as battery backup, standby, and emergency power. While storage at the factory or distribution center is also important during the transportation process, these areas are not addressed in this paper. Degrees Celsius and degrees Fahrenheit are expressed in the text simply as C or F.
Why is proper storage important?
Batteries are electrochemical devices, and the chemical reactions determine how they work and how they fail. Even in storage, these reactions take place and cause what is known as self-discharge. This causes the battery to lose capacity, and the amount is dependent upon factors such as storage time, ambient temperature, humidity, and chemistry. This loss in capacity can be reversed by applying a freshening charge to the battery. There are other losses that may occur when a battery is in storage that are not reversible. This is especially true if the battery is stored for too long a period.
Receiving the Battery.
Don’t assume that the battery has been shipped from the factory in a fully charged state, as this is sometimes not the case. It is a good idea to unpack the battery as soon as it arrives for several reasons, even when it is not due for immediate installation. Obviously, it needs to be inspected for any physical manufacturing defects, electrolyte leakage, or shipping damage. The carrier is not going to honor a damage claim that is filed several weeks after the battery has been delivered. Any shipping damage should be noted on the bill of lading and a claim filed with the shipper. If a manufacturing defect is suspected, then record the date of receipt, the defect(s), and notify the manufacturer immediately. It is also a good idea when unpacking to check that all the battery accessories are with the shipment. One does not want to temporarily store the battery and find out just before it is to be installed that parts are missing.
Unpacking.
With VRLA batteries, depending upon the weight of the battery units, they may be shipped double stacked on a pallet. This may save on shipping costs, but it does not do anything for easy battery inspection, testing, handling, or facilitating recharging. If the battery is to be stored in a hostile environment, or for a period that could possibly require a freshening charge, it is recommended that the shipment be broken down and single stacked on wooded or composite pallets. If additional pallets are introduced, care should be taken to inspect them for any nails or sharp objects that could possibly damage a battery unit. It is often stated that batteries should not be placed on concrete. This is a myth based on older battery technology when either glass or rubber containers were used. The glass containers were encased in wooden crates and the moisture from the cement floor could cause the wood to swell and fracture the glass. The rubber containers had a high carbon content and were somewhat porous. It was possible for a short to occur between the battery and the floor if the concrete was moist. Because of the advanced technology of today’s batteries and the types of electrically insulted containers used, the old reason for not storing on concrete no longer applies. However, I would still advise against this for a different reason. In many cases, a concrete floor, even if swept clean, may be uneven or contain minor imperfections, dirt and dust that could damage the bottom of a battery container.
Unpacking also allows for the checking of the individual unit manufacturing date codes. This is extremely important because it will likely indicate when the battery last received a charge. It is quite common for batteries of differing date codes to be shipped in the same batch. This is more applicable to batteries shipped from a reseller’s facility. It may be necessary to contact the manufacturer so that the date code can be deciphered or even located. If the batteries are over three to six months old, depending on the plate composition, ask the supplier to furnish the freshening charge details. If this information is not available, do not take ownership of the batteries. Also ask that the warranty start on the date the batteries were received from the shipper rather than the manufacturing or other date.
Voltage Checks.
Another thing to consider is that batteries are not usually 100% charged coming off the factory formation process. If the batteries are to be stored, it is a good idea to check the individual cell/unit voltages. This will give an indication of the State of Charge (SoC) of each cell/unit. The Open Circuit Voltage (OCV) of a battery that is not connected to a load is directly related to the Specific Gravity (SG) of the electrolyte. This can easily be calculated by adding the constant of 0.845 to the SG.
For example, for a 2V cell with an SG of 1.250, the fully charged OCV would be 0.845 + 1.250 = 2.095V. For a 6 cell, 12V unit with an SG of 1.300, the OCV would be (0.845 + 1.300) x 6 cells = 12.87V. If we use the above calculation as a baseline, then we can approximate the SoC of a battery cell/unit. Figure 1 below, is an example for a typical 12V, 1.300 SG VRLA battery. The author has approximated the OCV readings. As the figure indicates, when the OCV decays to about 12.6V, it is approximately 80% charged, and when it decays to about 12.35V, it is only 60% charged.
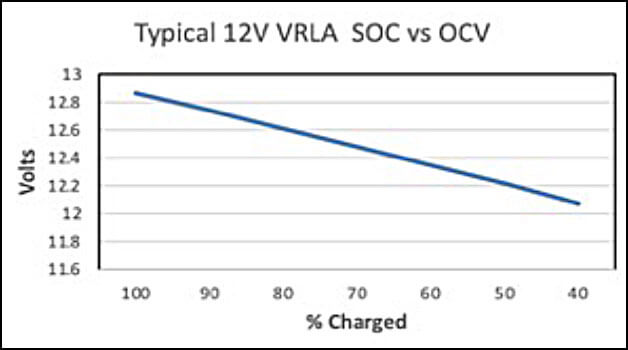
Figure 2 below shows the same type of data for a typical 1.250 SG VLA 2V cell.
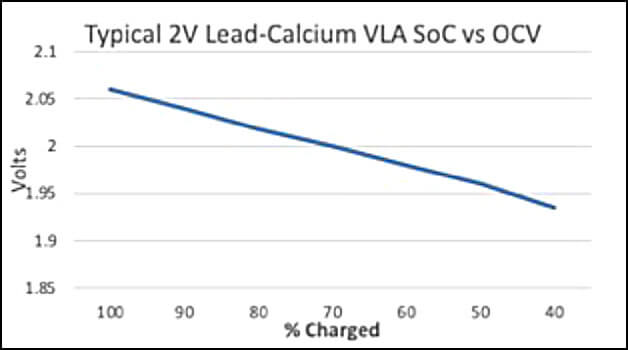
If the battery SoC is below 80%, then it is recommended that the battery be given a freshening charge as soon as possible. If the SoC is below 60%, then the battery may have some sulfation issues that may or may not be reversible.
Storage Location.
Ideally, batteries should be stored indoors in a clean, dry, cool, ventilated location that is away from foot traffic yet easily accessible.
Clean.
Dust and dirt are enemies of a battery and can cause shorts which could discharge the battery. If it is not possible to store the battery in a clean area, such as a facility under construction, then precautions should be taken to keep the battery dust and debris free.
Dry.
High Relative Humidity (RH) can cause problems for a battery by causing moisture to condense on the battery, leading to the possibility of short circuits. The ideal RH in the storage area should be around 35% to 55%, but the absolute maximum should be 90%. With high RH, one must be aware of condensation on the batteries, and it is not recommended that batteries should be covered with plastic sheeting. Low RH can cause a buildup of static electricity. In severe cases, this may cause an explosion internal to the battery.
Cool.
Temperature had a significant effect on battery storage, and this is discussed further on in this paper. Suffice to say, now that the ideal temperature of the storage location should be between 15C (60F) and 30C (85F). High temperature can significantly increase self-discharge. It should be noted that if batteries are stored in a warehouse on storage racks that may be several meters (feet) high, temperature stratification can occur where the upper batteries may be at a higher temperature than the lower ones.
Ventilation.
Since the batteries, particularly the VLA type, will vent hydrogen if they are given a freshening charge, the storage location should have sufficient natural or forced air ventilation to dissipate any hydrogen gas released. The concentration of hydrogen in air should be kept at less than 1%.
Safety.
There are two elements of personnel safety that can be involved with battery storage, and these are physical and electrical.
Physical Safety.
Batteries can be heavy and awkward to handle. The proper Personal Protective Equipment (PPE) should be worn that is appropriate for the task. This can include, but is not limited to, the following where appropriate. Good reference sources are IEEE Std 4841 and Std 11872.
· Safety footwear.
· Safety glasses with side shields of face shields.
· Acid resistant and electrically rated gloves.
· Acid resistant aprons.
Electrical Safety
When it is necessary to apply a freshening charge, one will be exposed to electrical shock hazard, and it should be treated just the same as working on an energized battery.
It is also necessary to have the following equipment and materials available.
· Appropriate arc rated clothing.
· Electrically insulated tools.
· Appropriate lifting devices.
· Acid neutralizing agent.
· Eye wash equipment.
Battery handling.
Most battery accidents occur when they are being handled3. When storing batteries, this can involve several handling operations. In the United States, the Occupational Safety and Health Administration (OSHA) does not specify battery lifting or moving equipment or methods; however, OSHA can impose penalties if personnel are injured in the workplace. The most common injuries reported are back injuries. In order to reduce injuries caused by lifting, the U.S. National Institute of Occupational Safety and Health (NIOSH)4 has developed a lifting equation designed to determine the safety of various lifting tasks. The NIOSH calculator is designed only for a single person, two-handed manual lifting. Consequently, most stationary battery handling falls outside the scope of NIOSH and will require some type of lifting device. Trial calculations by the author indicate that, in general, it is unsafe for one person to lift a battery unit weighing more than 23kg (50 lbs.) above 3 feet. Where necessary, use the manufacturer supplied lifting devices for lifting and moving batteries. If a mechanical battery lifting device such as a pallet stacker is used, ensure that all metallic parts that could come in contact with the battery terminals are insulated. For more detailed information on VRLA battery handling, refer to IEEE Std. 1187 – 2013, Annex F2.
Temperature effects on battery storage.
A lead-acid stationary battery is sensitive to temperature. The optimum storage temperature is 20C to 25C (68F to 77F) and extrusion beyond this window will affect self-discharge and consequently the period between freshening charges. Batteries should not be stored in direct sunlight or be subject to wide ambient temperature fluctuations.
The Arrhenius equation, which was developed in 1889 by the Nobel Prize winning Swedish scientist Svante Arrhenius, states that an increasing temperature produces an exponential increase in reaction rate. This is important when applied to a stationary battery in storage. For example, a battery that is stored at a temperature of 10C (18F) above the ideal 25C (77F) will self-discharge twice as quickly as a battery being stored at the ideal temperature. The same will apply to a lowering of the self-discharge rate of batteries stored at a temperature below the nominal.
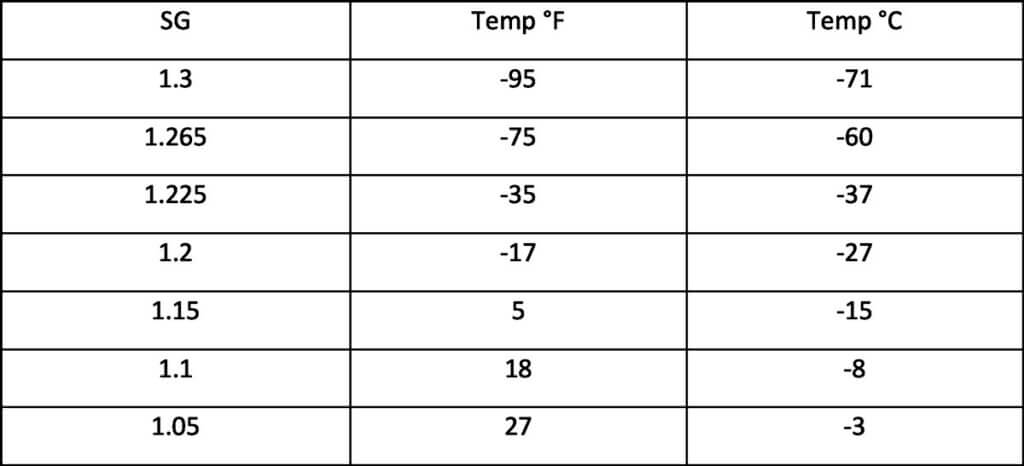
Even though storage below the ideal storage temperature will decrease the self-discharge rate, one must be careful that the storage temperature is not so low as to cause the electrolyte to freeze. The freezing point of the sulfuric acid electrolyte is dependent upon the concentration of the sulfuric acid. The higher the concentration, the lower the freezing point. Most stationary batteries have an SG between 1.215 and 1.32, but this is the case, only for a fully charged battery. As the battery becomes discharged, the SG decreases and consequently the freezing point rises. Water freezes at 0C (32F), so unless the battery is completely discharged, the freezing point will be some value below this. A lower temperature will greatly slow down the self-discharge, but if the SG of the electrolyte has decreased sufficiently, the freezing point will be reduced. Figure 3 below gives an indication of how the SG relates to temperature. As can be seen, if the specific gravity falls, the freezing point of the electrolyte is raised, i.e. it will increase towards 0C (32F).
Freezing point of Electrolyte
When to apply a Freshening Charge.
Battery storage times and recharge requirements differ from manufacturer to manufacturer. This is because these values are dependent upon variables such as the chemical composition of the battery and the SG. Consequently, manufacturers will make recommendations for each different product. The most common recommendations are based upon a storage temperature of 20C – 27C (68F – 80F). Consequently, for storage extrusions beyond this window, adjustments will have to be made to the timing of a freshening charge.
The most common recommendation for freshening charges is after 6 months storage for lead-calcium product and 3 months for lead-antimony and lead selenium product. However, these are based upon a fully charged battery, and as stated previously, there is no guarantee that the battery is fully charged when received from the shipper. The time for a freshening charge is when the battery SoC has decreased to 80%. See Figures 1 and 2 above.
Specific Gravity
If SG rather than voltage measurements are being used to determine the SoC, Figures 1 and 2 can still be used by adding 0.845 to the SG value. That will give the voltage reading that equates to the SoC.
The Freshening Charge
A freshening charge for a stored battery is pretty much the same as applying the freshening charge upon battery installation5. The manufacturer’s freshening charge recommendations should be considered. It will usually be somewhere between the recommended float and equalize voltages, applied for about 96 hours for VLA and 24 hours for VRLA, and for such a time that the charge current has stabilized for a 3-hour period. If the stored battery has been determined to be below 80% charged but above 60% charged, then it may be required that the battery be given a freshening charge at the recommended equalize voltage or higher. This may help to reverse any sulfation that may have developed. If the battery is below 60% SoC, it is possible that the equalize charge may be insufficient to overcome any sulfation.
If there are any charge current limitations for the battery, as is often the case with VRLA batteries, care must be taken not to exceed this value.
It is also important to compensate the freshening charge voltage for the temperature of the battery at the time of the charge. Temperature compensation figures are supplied by the manufacturers and are usually expressed in millivolts per degree C or F per cell. For example: 3mV/°C (1.7mV/°F) per cell above or below 25°F (77°F). This value would be subtracted from the charge voltage for a battery that is above 25°F (77°F) and added to the charge voltage for a battery that is below 25°F (77°F). For example, a 60-cell battery is stored in a location where the ambient temperature is 90°F. This is 13°F above the 77°F nominal. If the temperature compensation factor for this battery is 1.7mV/°F per cell, then the calculation would be as follows:
13°F x 1.7mV = 22.1mV/cell. If the recommended freshening charge voltage is 2.3V/cell, then 22.1mV would be subtracted from the recommended 2.3V/cell, which would result in a temperature compensated voltage of 2.2779V/cell. Multiplied by 60 cells this would mean that the 60 cell, 120-volt nominal battery should be charged at 136.7V.
The voltage and capacity of the charger that is being used for the freshening charge will determine how the stored battery is connected and segmented for charging. For example, if only a nominal 120-volt charger is available and there are 240 cells to recharge, then there are several choices as to how the 240 cells can be connected to the charger. Sixty cells can be connected in series and charged separately from the remainder, or two, three, or four sixty cell series strings can be connected in parallel. This will be dictated by the capacity of the charger and its ability to supply sufficient current to charge the battery in the allocated time. Ensure that the size of the connectors and the clamps that are used to connect the charger to the battery are used as inter-cell connectors and are of sufficient size to carry the charging current.
Note. If the overall battery string voltage is greater than 100V nominal, it is advisable to segment the battery so that each connected string is below 100V nominal. This will greatly reduce the chance of a severe arc flash incident and keep the voltage below the threshold voltage determined by NFPA 706.
When the battery is taken off the freshening charge, it should be allowed to rest for a period of approximately 24 hours on open circuit before individual unit voltage and or SG readings are taken. All the charging details, including the before and after voltage and SG readings, the charge voltage, the period of the charge and the resting time, should be recorded and kept with the battery paperwork. This may be required for warranty reasons. It is also a good idea to put a label on each battery unit indicating the date of the completion of the freshening charge.
Summary.
If a battery is to be kept in temporary storage, it is extremely important that the battery be stored properly and given freshening charges as necessary. Failure to do so will damage and degrade the battery, causing it to age faster, shorten the life, lower the performance, and cause problems with any future warranty claims. Knowledge of the actions that should be taken and how to perform these actions is extremely important. Do not hesitate to enter into a dialog with the manufacturer or supplier, even if short term storage is involved. If the user can prove that the battery was stored and maintained in the correct manner, the manufacturer or supplier may extend the warranty period to the date the battery is placed in service, even though this may exceed the normal allowable period from the shipping date. Also, do not be afraid to negotiate a new warranty with the manufacturer or supplier7.
References.
- IEEE Std 484 Latest Issue. IEEE Recommended Practice for Installation Design and Installation of Vented Lead-Acid Batteries for Stationary Applications. The Institute of Electrical and Electronics Engineers, Inc.
3 Park Avenue, New York, NY 10016-5997, USA - IEEE Std 1187-Latest Issue. IEEE Recommended Practice for Installation Design and Installation of Valve-Regulated Lead-Acid Batteries for Stationary Applications. The Institute of Electrical and Electronics Engineers, Inc. 3 Park Avenue, New York, NY 10016-5997, USA
- Byrne, J. Allen. Battery handling into and at the installation site. Are you doing it right? Proceedings of Battcon 2009.
- Applications Manual for the Revised NIOSH Lifting Equation. U.S Department of Commerce, Technology Administration, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161.
- Byrne, J. Allen. The proper charging of stationary lead-acid batteries. Proceedings of Battcon 2010
- NFPA 70E. Latest Issue. The National Electric Safety Code. National Fire Protection Association (NFPA). 11 Tracy Drive, Avon, MA 02322
- Ashton, Curtis and Byrne, J. Allen. Data gathering to ensure battery warranties are honored. Proceedings of Battcon 2014
Need a Copy of What You Just Read?
Download the PDF version of this white paper for future reference.

